- Product
- Temperature Measurement
- Temperature Humidity Measurement
- Wireless Measurement
- Water Quality Meter
- Flow Measurement
- Accessory product
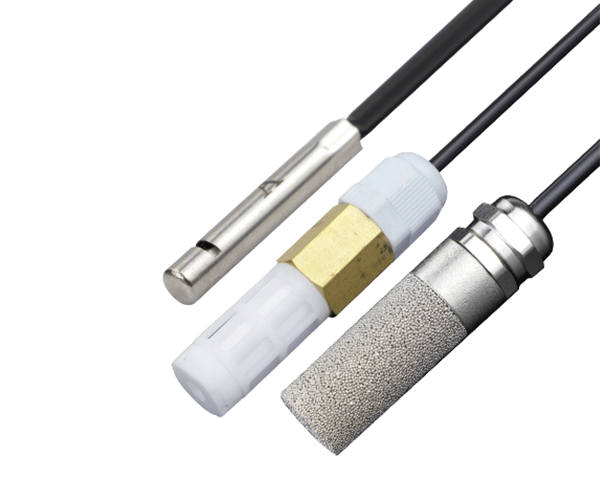
- Temperature Sensor Temperature Transmitter Thermometer
-

Temperature Sensor

Temperature Transmitter

Thermometer
- Temperature humidity sensor Temperature humidity transmitter Thermometer hygrometer
-

Temperature humidity sensor

Temperature humidity transmitter
Thermometer hygrometer
- Wireless Temperature Transmitter Wireless temperature humidity transmitter Wireless Thermometer
-

Wireless Temperature Transmitter

Wireless temperature humidity transmitter
Wireless Thermometer
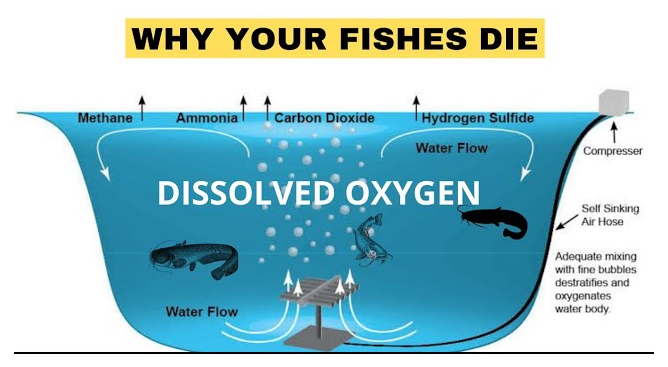
- pH Meter Conductivity Meter Dissolved Oxygen Meter Residual chlorine meter Turbidity meter Multiparameter
-

pH Meter

Conductivity Meter

Dissolved Oxygen Meter

Residual chlorine meter

Turbidity meter

Multiparameter
- Solution
- Smart Home Smart agriculture New Energy Vehicle Water Treatment Food Beverage Electricity Energy Medicine Cold Chain Transportation Office Industrial Equipment Oil and Gas
-

Smart Home
Smart agriculture

New Energy Vehicle

Water Treatment

Food Beverage

Electricity Energy

Medicine

Cold Chain Transportation
Office Industrial Equipment

Oil and Gas
- Service
- Support
- IOT
- About us