Are you looking to understand pH meters inside and out? Whether you're a student, a lab technician, or someone working in quality control, this guide is designed for you. By the end of this article, you will have a clear understanding of what a pH meter is, how it works, the different types available, and a practical step-by-step guide on how to use and calibrate one. Most importantly, we'll help you figure out exactly how to choose the right pH meter for your specific application.
1. What is a pH Meter?
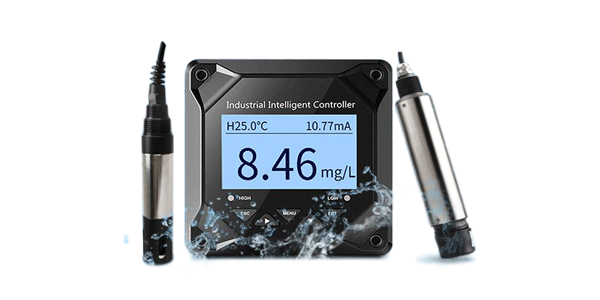
A pH meter is a scientific instrument that measures the hydrogen-ion activity in water-based solutions, indicating its acidity or alkalinity expressed as pH. It is an essential tool in many laboratories, industries, and even for some home use cases.

The pH scale ranges from 0 to 14, with 7 being neutral. A pH less than 7 is acidic, while a pH greater than 7 is basic or alkaline. pH meters provide a much more precise and accurate measurement than pH strips or indicator solutions.
2. How Does a pH Meter Work? (The Principle)
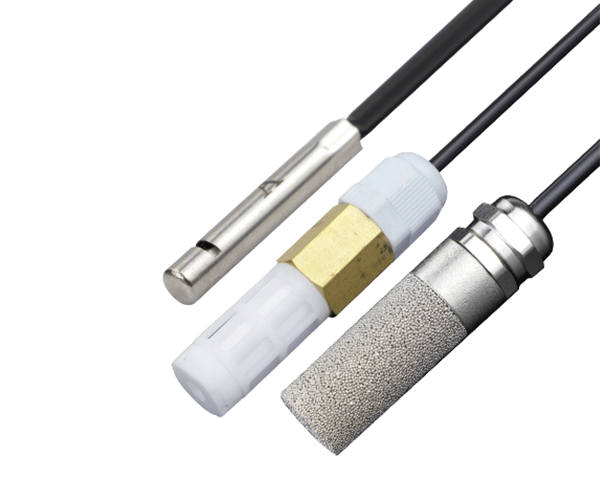
A pH meter works by measuring the electrical potential (voltage) between two electrodes: a pH electrode (glass electrode) and a reference electrode. Often, these are combined into a single combination electrode.
- The thin glass bulb at the tip of the pH electrode is sensitive to hydrogen ions.
- When immersed in a solution, a potential difference develops across the glass bulb relative to the stable potential of the reference electrode.
- This potential difference is measured by the meter's high-impedance voltmeter.
- The meter then converts this millivolt signal into a corresponding pH value, which is displayed on the screen.
3. Key Components of a pH Meter
Understanding the main parts will help you use and maintain your device properly.
- Electrode: The most critical part, usually a combination electrode, which houses both the measuring and reference cells.
- Reference Junction: A porous plug that allows for electrical contact between the reference cell and the test solution.
- High-Impedance Amplifier: The "brain" that accurately measures the high-resistance electrical signal from the electrode.
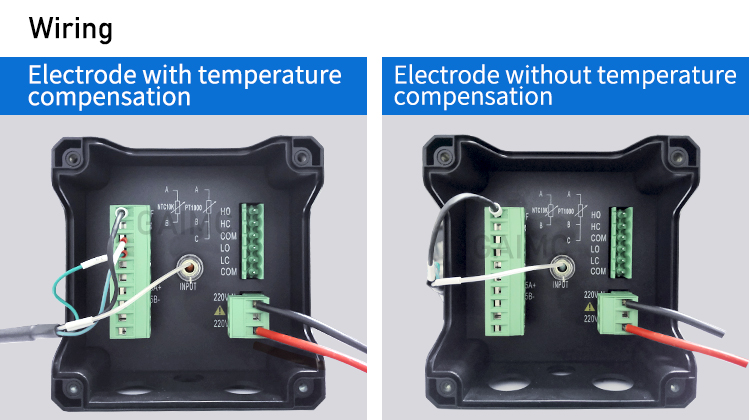
- Temperature Sensor (ATC): Many modern meters have Automatic Temperature Compensation (ATC) to adjust for temperature changes that affect pH readings.
- LCD / Display: Shows the pH reading, temperature, and other data.
4. Types of pH Meters & Their Applications
Choosing the right type depends heavily on your application.
| Type | Best For | Pros & Cons |
|---|---|---|
| Online pH Meters | Laboratories, quality control labs, research facilities. | Pros: High accuracy, stable, multiple features. Cons: Not portable, higher cost. |
| Portable / Handheld pH Meters | Field testing, aquaculture, hydroponics, environmental monitoring. | Pros: Battery-powered, rugged, on-the-go use. Cons: Slightly less accurate than benchtop models. |
| Pen Testers | Basic water testing, home brewing, aquariums, education. | Pros: Very affordable, compact, simple. Cons: Lower accuracy, fewer features. |
For example, our online water quality meters are widely used by local environmental agencies for water quality testing and by hydroponic farms for nutrient solution management.

5. How to Calibrate a pH Meter
Calibration is crucial for accurate results. It involves adjusting the meter to known standard buffers.
Step-by-Step Guide:
- Prepare Buffers: Use fresh, pH 7.01 and pH 4.01 (for acidic range) or pH 10.01 (for basic range) calibration buffers.
- Rinse Electrode: Rinse the electrode with distilled water and blot dry with a soft tissue.
- Immerse in pH 7 Buffer: Place the electrode in the pH 7.01 buffer, stir gently, and press "Calibrate" once the reading stabilizes.
- Rinse and Second Point: Rinse again, then place the electrode in the second buffer (e.g., pH 4.01), and calibrate.
- Verification: Rinse and measure the first buffer again to verify accuracy. The reading should be very close to 7.01.
Pro Tip: For guaranteed accuracy, consider our professional pH meter calibration service available in [Your City].
6. pH Meter Maintenance & Storage
Proper care extends the life of your electrode.
- Cleaning: Clean the electrode regularly with a solution suitable for the contaminants (e.g., protein, oil).
- Storage: Never store in distilled water! Always store the electrode in a storage solution or pH 4 buffer to keep the glass bulb hydrated.
- Handling: Avoid scratching the delicate glass bulb. Do not let it dry out.
7. How to Choose the Right pH Meter
Selecting the best pH meter depends on your specific requirements. Ask yourself these questions:
- What is my application? (Lab research, field testing, educational demo?)
- What level of accuracy do I need? (e.g., ±0.001 pH for research, ±0.1 pH for general use).
- Do I need portability?
- What is my budget?
- What features are important? (ATC, data logging, GLP compliance)?
Still Unsure Which pH Meter is Best for You?
Our online experts at GAIMC are here to help. We understand the needs of industries in your region and can recommend the perfect instrument for your application and budget.
Get a Free Consultation & Quote8. Conclusion
A pH meter is a fundamental instrument for measuring acidity and alkalinity with high precision. From understanding its basic principle to proper calibration and maintenance, using it correctly is key to obtaining reliable data. Whether you are in a high-tech lab in Your City or monitoring water quality in the field, choosing the right pH meter for your needs is essential for success.