Master Vaccine Safety: The Complete Guide to Cold Chain Temperature Monitoring
Are you responsible for maintaining vaccine efficacy and safety throughout the cold chain? Precise temperature monitoring is critical for protecting public health and ensuring biological product integrity. In this guide, you'll discover how advanced temperature monitoring systems work, learn about ultra-low temperature requirements for vaccine preservation, and understand the technology that ensures vaccine potency from manufacturing to administration.
Why Vaccine Cold Chain Monitoring is Critical for Public Health?
Vaccine cold chain management represents a vital component of modern healthcare infrastructure—it's about preserving biological efficacy, ensuring patient safety, and maintaining public trust in immunization programs.
With economic development and rising quality of life standards, drug safety and quality concerns have gained significant attention, driving continuous growth in the vaccine cold chain transportation industry. Vaccines are biological products exceptionally sensitive to temperature variations. When transportation and storage temperatures fall outside required ranges, vaccines can lose potency or become completely ineffective, compromising both safety and protective capabilities. The primary mission of vaccine cold chain systems is maintaining appropriate temperature ranges throughout production, transportation, and storage to preserve vaccine activity and stability, ultimately safeguarding public health.

Real-World Vaccine Cold Chain Monitoring Case Study
This application demonstrates comprehensive temperature monitoring throughout vaccine cold chain transportation to ensure product quality and therapeutic effectiveness.
Critical Requirements
The deep cold chain environment demands extreme temperature resilience, with minimum temperatures reaching -80°C requiring specialized cold-resistant sensors. Exceptional temperature measurement accuracy is mandatory since even minor deviations can cause vaccine failure. High corrosion resistance ensures cable stability in extreme environments, while compact spacing necessitates convenient wiring solutions for limited installation areas.
Advanced Technical Solution
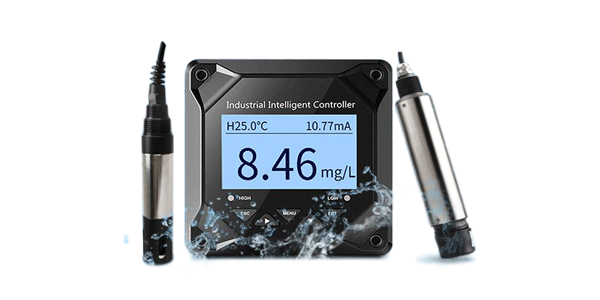
Our solution employs ultra-low temperature series Pt1000 chips capable of reaching -196°C, featuring rapid response times, exceptional accuracy, excellent long-term stability, superior thermal shock resistance, and ultra-wide operating temperature ranges specifically designed for cryogenic applications. This advanced technology significantly enhances safety and reliability for sensitive biological products.
Specialized Cable Infrastructure
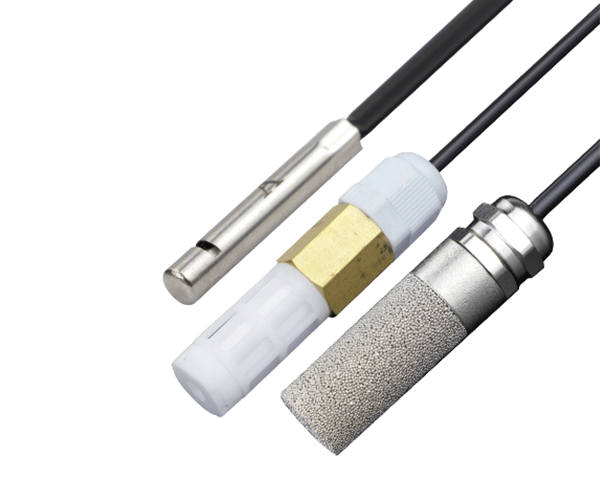
We utilize PFA low-temperature resistant flat cables rated for -196°C, featuring space-efficient designs that minimize spatial requirements while simplifying installation. The cable exterior incorporates Halar ECTFE anti-corrosion coating, substantially improving corrosion resistance for long-term reliability in challenging environments.
Key Features of Vaccine Cold Chain Monitoring Systems
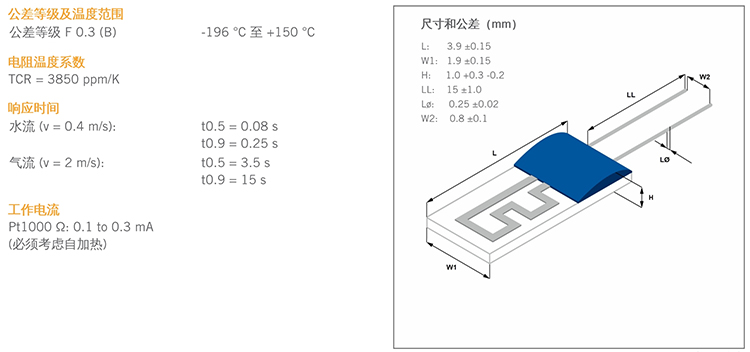

Ultra-Low Temperature Sensor Technology
Our Pt1000 chip sensors deliver laboratory-grade accuracy in extreme cryogenic environments, maintaining precision from -196°C to higher temperature ranges with exceptional stability and repeatability for reliable vaccine monitoring.
Advanced Cable Materials
Specialized PFA flat cables provide unparalleled flexibility and durability in ultra-cold conditions, while the compact flat design optimizes space utilization in crowded cold chain equipment and transportation containers.
Enhanced Corrosion Protection
Halar ECTFE coating offers superior chemical resistance against cleaning agents, moisture, and environmental contaminants, ensuring long-term operational integrity in demanding cold chain environments.
Rapid Response Monitoring
Advanced thermal coupling and sensor design enable immediate temperature change detection, providing real-time alerts for temperature excursions that could compromise vaccine efficacy.
Frequently Asked Questions (FAQ)
What temperature range do most vaccines require?
Most vaccines require strict temperature control between 2°C and 8°C for refrigerated products, while some modern vaccines (particularly mRNA varieties) require ultra-cold storage at -60°C to -80°C, with specific requirements varying by manufacturer and vaccine type.
How quickly can temperature excursions affect vaccine efficacy?
Some temperature-sensitive vaccines can begin degrading within minutes of exposure to temperatures outside their specified range. The exact timeframe varies by vaccine formulation, but immediate action is always recommended when excursions occur.
What certification standards apply to vaccine cold chain monitoring?
Key standards include WHO PQS (Performance Quality Safety), CDC vaccine storage guidelines, EU GDP (Good Distribution Practice), and various national regulatory requirements that mandate specific temperature monitoring and documentation protocols.
How often should cold chain sensors be calibrated?
For vaccine applications, we recommend quarterly verification and annual formal calibration. High-usage or critical applications may require more frequent calibration to ensure measurement integrity for sensitive biological products.
Can monitoring systems integrate with existing inventory management?
Absolutely. Modern cold chain monitoring systems typically feature API integrations, cloud connectivity, and compatibility with common inventory management platforms, enabling seamless data sharing and comprehensive supply chain visibility.
What backup systems protect against power failures?
Comprehensive systems include battery backups, cellular communication alternatives, data logging continuity features, and often secondary monitoring systems to ensure uninterrupted temperature surveillance during power disruptions or equipment failures.
Ready to Secure Your Vaccine Cold Chain?
Get professional temperature monitoring solutions for critical vaccine storage and transportation.