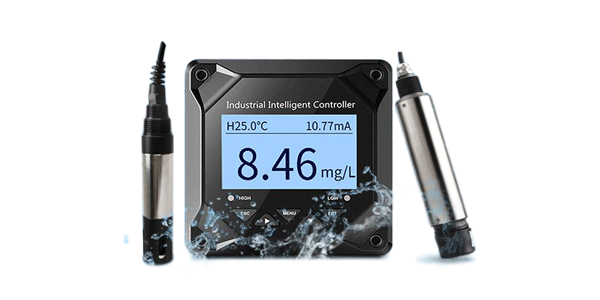
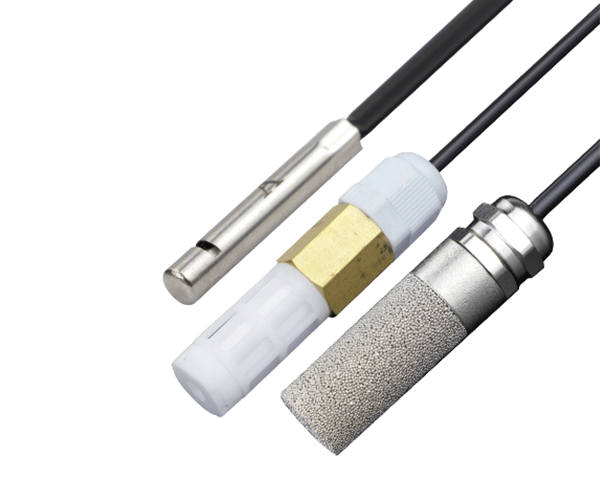
If you work with water quality monitoring in industrial, environmental, or laboratory settings, you've likely encountered conductivity probes. But do you truly understand how they work and why they're so critical to your operations? In this comprehensive guide, you'll discover the fundamental principles behind conductivity measurement technology, learn about different probe types and their applications, and understand how to select the right conductivity probe for your specific needs. By the end, you'll be equipped with the knowledge to optimize your water quality monitoring processes and make informed decisions about your measurement equipment.
The Fundamental Principle: What Conductivity Measures
Conductivity, in the context of water quality, refers to the ability of water to conduct an electrical current. This property directly correlates with the concentration of ions dissolved in the water. The more dissolved salts and inorganic materials present, the higher the conductivity reading. A conductivity probe essentially measures this ionic content by passing an electrical current through the water and detecting how easily that current flows.
Core Components of a Conductivity Probe
Every conductivity probe consists of several key components that work together to deliver accurate measurements:
Electrodes
Typically two or four electrodes made of durable, corrosion-resistant materials like stainless steel, graphite, or platinum.
Cell Constant
A defined geometrical parameter determined by the distance between electrodes and their surface area.
Temperature Sensor
Most modern probes include a thermistor to compensate for temperature variations, as conductivity is highly temperature-dependent.
Probe Body
Constructed from chemically resistant materials to protect internal components from harsh measurement environments.
How the Measurement Process Works
The probe is immersed in the solution to be measured.
The instrument applies an alternating current (AC) voltage to the electrodes.
The resulting current flow between the electrodes is measured.
The instrument calculates conductivity based on the current, applied voltage, and the probe's cell constant.
Simultaneously, the temperature is measured and used to standardize the reading to a reference temperature (typically 25°C).
Types of Conductivity Probes and Their Applications
Two-Electrode Probes
Ideal for low to medium conductivity ranges, these probes feature two electrodes that both apply and measure the current. They're cost-effective and suitable for many general-purpose applications, including drinking water monitoring and basic laboratory measurements.
Four-Electrode Probes
Using separate pairs of electrodes for current application and voltage measurement, four-electrode probes eliminate polarization effects and provide superior accuracy in high-conductivity applications. They're essential for seawater monitoring, wastewater treatment, and concentrated industrial solutions. Our industrial conductivity probes utilize this technology for challenging environments.
Electrodeless (Toroidal) Probes
These probes use electromagnetic induction rather than direct electrode contact, making them virtually immune to fouling and corrosion. They excel in dirty wastewater, slurries, and highly corrosive chemical applications where traditional electrodes would quickly degrade.
Factors Affecting Conductivity Measurement Accuracy
Temperature
Conductivity increases approximately 2% per °C - temperature compensation is essential.
Polarization
Electrode surface effects that can distort measurements, especially in high-conductivity solutions.
Fouling
Coating or debris buildup on electrodes alters the cell constant and reduces accuracy.
Cable Length
Long cables can introduce capacitance that affects measurement stability.
Industry Applications of Conductivity Measurement
Water Treatment
Monitoring reverse osmosis efficiency, detecting contaminant ingress, and controlling chemical dosing.
Pharmaceutical
Ensuring water purity in USP purified water and Water for Injection systems.
Food & Beverage
Controlling product consistency, cleaning processes, and water quality.
Power Generation
Monitoring boiler feedwater, steam purity, and cooling water systems.
For industry-specific challenges, explore our comprehensive water quality solutions.
Sources and Further Reading
This article draws upon established principles of electrochemistry and water quality measurement. Technical references include:
Frequently Asked Questions
Why is temperature compensation necessary in conductivity measurement?
Conductivity is highly temperature-dependent, with values increasing approximately 2% per degree Celsius. Without temperature compensation, measurements taken at different temperatures cannot be accurately compared. All modern conductivity instruments include automatic temperature compensation to standardize readings to 25°C.
How often should conductivity probes be calibrated?
Calibration frequency depends on the application and required accuracy. For critical processes, monthly calibration is recommended. For general monitoring, quarterly calibration may suffice. Always calibrate when changing solutions, after cleaning, or if readings become unstable. Use appropriate conductivity calibration solutions for best results.
What's the difference between conductivity and TDS measurements?
Conductivity measures the water's ability to conduct electricity, while TDS (Total Dissolved Solids) estimates the total concentration of dissolved substances. TDS is typically calculated by multiplying conductivity by a factor (usually 0.5-0.7). Conductivity provides the direct measurement, while TDS represents an inferred value.
How can I prevent fouling on my conductivity probe?
Regular cleaning with appropriate solutions, using probes with self-cleaning mechanisms or special coatings, and selecting electrodeless probes for severely fouling applications can minimize fouling issues. For persistent fouling problems, consult our technical support team for application-specific recommendations.
Get the Right Solution for Your Conductivity Measurement Needs
Choosing the appropriate conductivity probe requires understanding your specific application, measurement range, and environmental conditions. GAIMC offers comprehensive water quality monitoring systems with precision conductivity probes designed for industrial and municipal applications.
Our technical experts can help you select from our range of water quality products or develop custom solutions for challenging applications.
Contact Our Measurement Experts Today